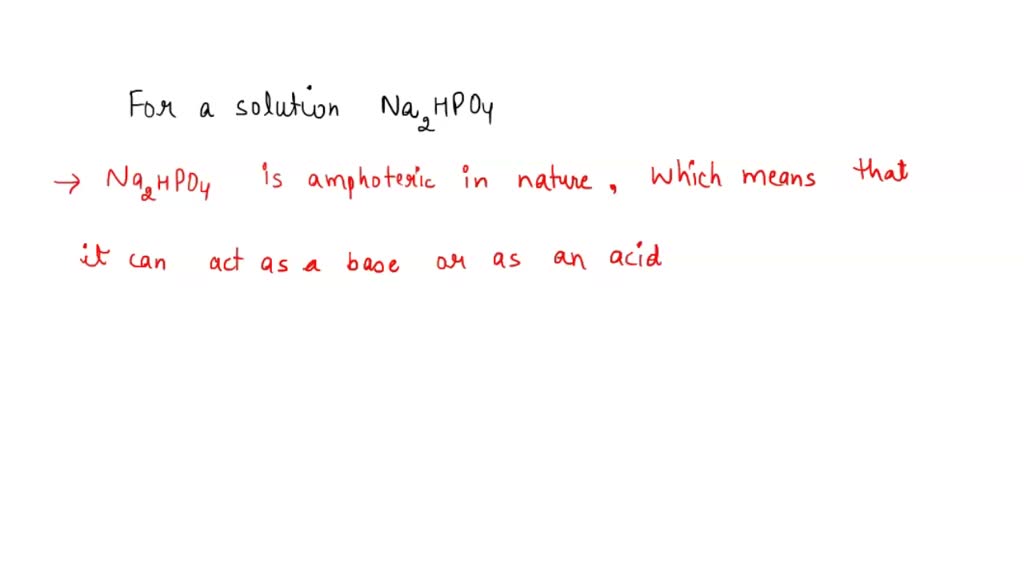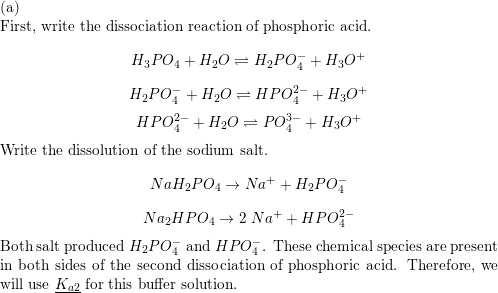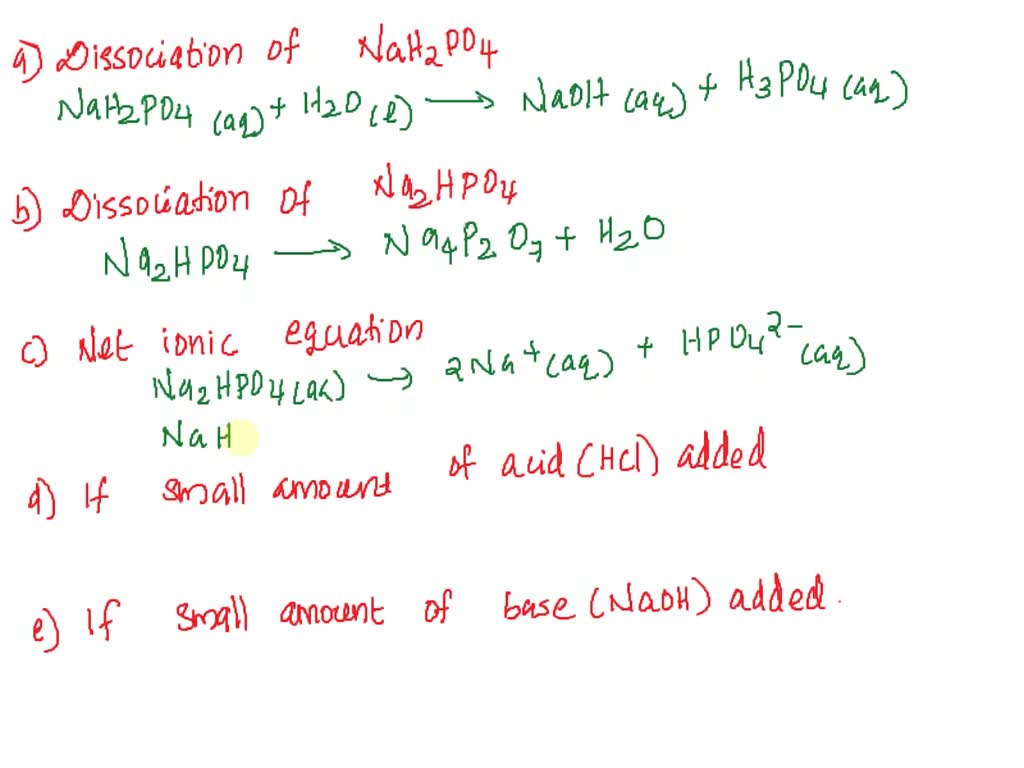
SOLVED: A buffer was made by mixing aqueous solutions of NaH2PO4 and Na2HPO4 together. This buffer is made by mixing two salts together. a. Write the balanced dissociation reaction for solid NaH2PO4

OneClass: Equal molar quantities of sodium hydroxide and sodium hydrogenphosphate (Na2HPO4) are mixed...

A buffer solution 0.04 M in Na2HPO4 and 0.02 M in Na3PO4 is prepared. The electrolytic oxidation of 1.0 milli - mole of the organic compound RNHOH is carried out in 100

If 2.5 moles each of H3PO4,NaH2PO4,Na2HPO4 and Na3PO4 are mixed together to form an aqueous solution, then the resulting pH is:Given values of Ka are: Ka1 = 10^-3 Ka2 = 10^-7 Ka3 = 10^-13

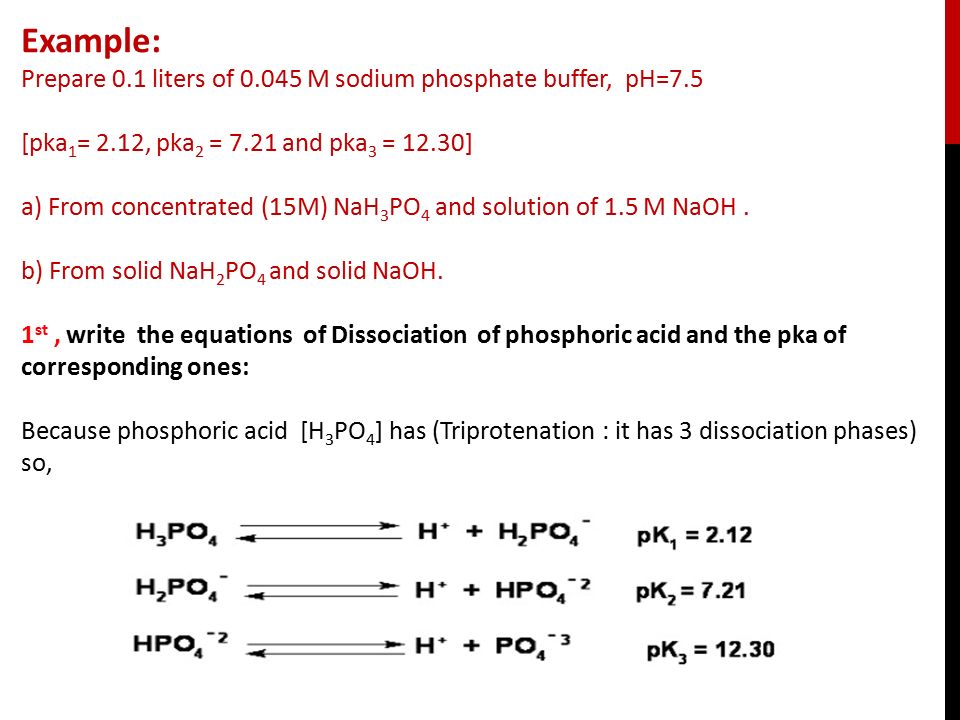
Calculate the pH of a buffer solution obtained by dissolving 25.0 g of KH2PO4(s) and 38.0 g of Na2HPO4(s) in water and then diluting to 1.00 L. | Homework.Study.com

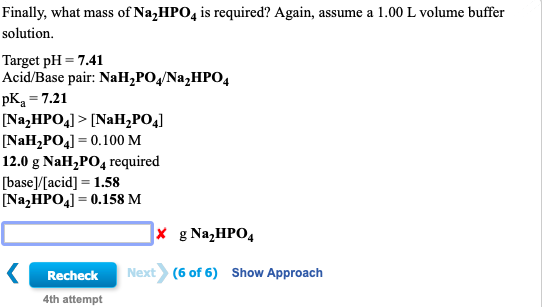
Finally, what mass of Na2HPO4 is required? Again, assume a 1.00 L volume buffer solution. Target pH = 7.49 - Brainly.com
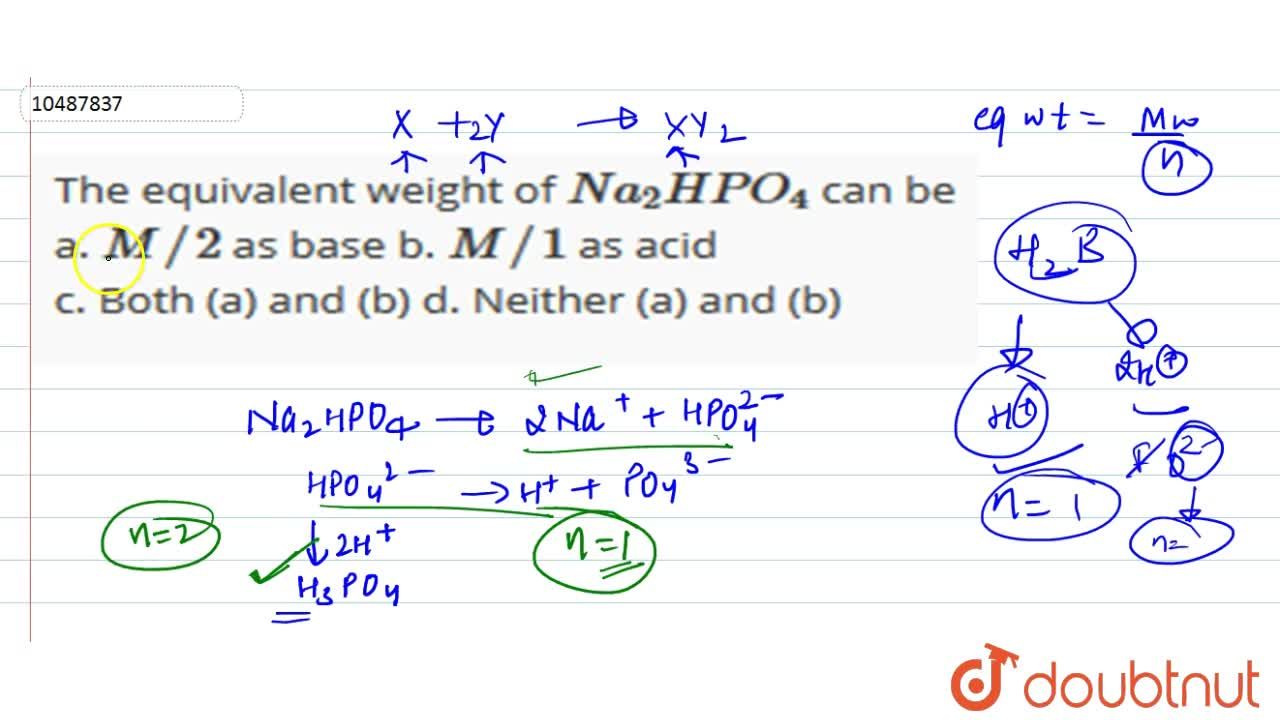
The equivalent weight of Na(2) HPO(4) can be a. M//2 as base b. M//1 as acid c. Both (a) and (b) d. Neither (a) and (b)


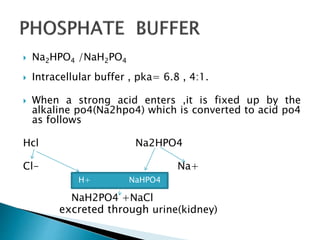
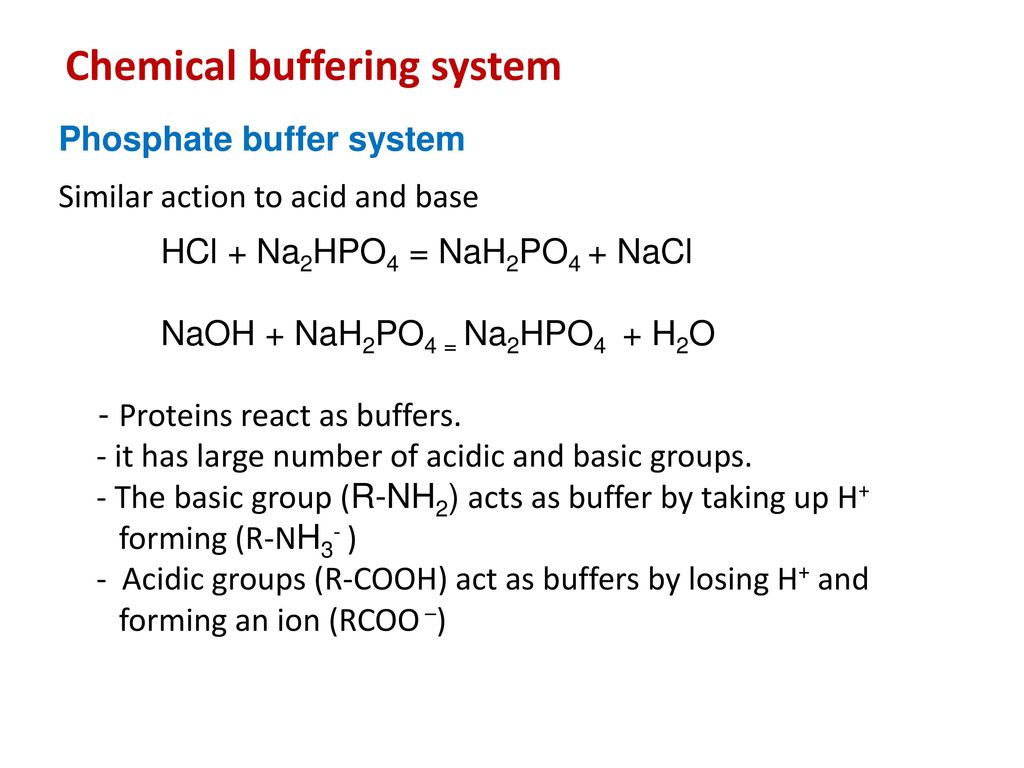
![An example of an acid salt is [CH(3)COONa//NaNO(3)//Na(2)HPO(4)//NaKCO(3)] An example of an acid salt is [CH(3)COONa//NaNO(3)//Na(2)HPO(4)//NaKCO(3)]](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/643651395_web.png)